Which of the Following Exists as a Polyatomic Molecule
It is also referred to as a radical. Are polyatomic elements.
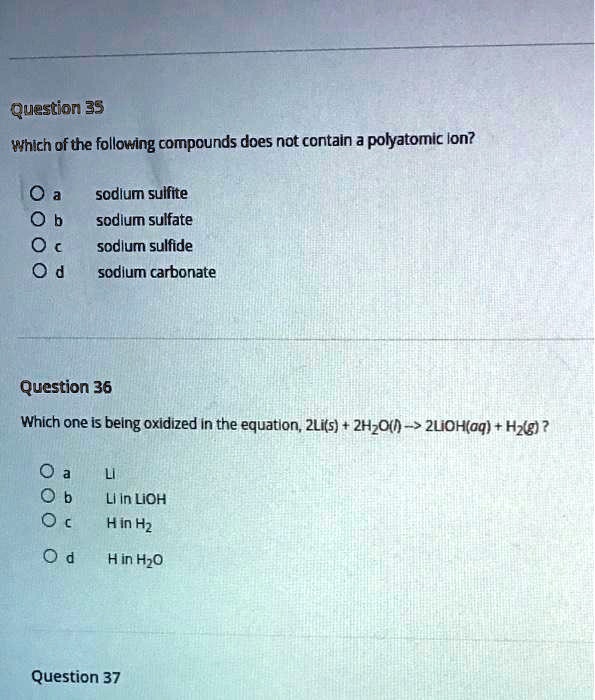
Solved Uestion 35 Which Of The Following Compounds Does Not Contain A Polyatomic Ion Sodlum Sulfite Sodium Sulfate Sodlum Sulfide Sodlum Carbonate Question 36 Which One Is Being Oxidized In The Equatlon Zlifs
A bond between two polyatomic ions.
. E a bond between two polyatomic ions. D a bond between a metal and a polyatomic ion. Ate ending in a.
Carbon phosphorus argon sodium neon. Any molecule that contains more than 2 atoms is polyatomic. See the answer See the answer done loading.
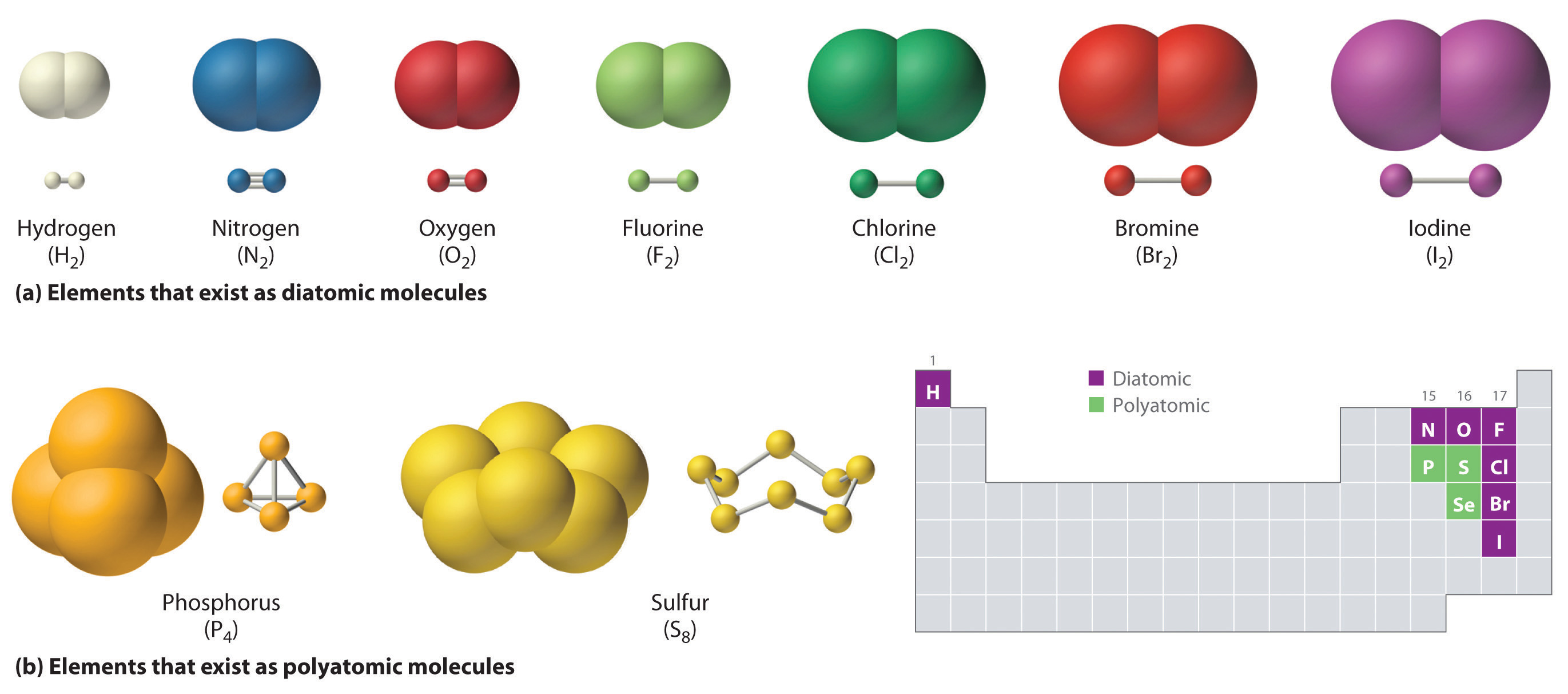
Polyatomic ions consist of ions that have charges that cancel each other. Information about various chemical compounds and elements. The elements found as diatomic molecules are hydrogen H element 1 nitrogen N element 7 oxygen O element.
And The PCl3 molecule has a tetrahedral electron geometry with three bonded atoms and one lone pair of electrons which gives it a trigonal pyramidal shape. It appears in diatomic form as O_2 which is a combination of two oxygen atoms. Because the molecule CCl4 has a tetrahedral shape the four CCl dipoles cancel which makes CCl4 a nonpolar molecule.
A the sharing of electrons between atoms. Which of the following elements exists as a diatomic molecule A neon B lithium C from BIO 3 at University of Caloocan City formerly Caloocan City Polytechnic College. A neon B carbon C phosphorus D lithium E krypton.
3 Give a possible molecular formula for the empirical formula of C3H5ClO. A number of elements are found in their elemental form as diatomic moleculesIn these molecules two atoms are joined by one or more covalent bonds forming a molecule with the general formula X 2. 19 Which of the following exists as a polyatomic molecule.
Diatomic molecules such as and undergo simple harmonic motion with frequencies that obey Hookes Law with the effective mass being half the atomic mass. Polyatomic molecules are electrically neutral groups of three or more atoms held together by covalent bonds. Chlorine Cl 2 Nitrogen N 2 and Hydrogen H 2 are diatomic gases.
Which of the following exists as a polyatomic molecule. And phosphorous P 4. Correct options are A and B Sulphur S 8.
The elements that have more than two atoms bonded by a covalent bond are referred to as polyatomic elements. Molecular compounds is composed of two or more covalently bonded non-metals. Oxygen exists as O2.
C a bond between a metal and a nonmetal. The elements which exist as groups of more than two atoms are called polyatomic elements. Hydrogen exists as H2.
Hydrogen nitrogen oxygen fluorine chlorine iodine bromine. Therefore it exists in its elemental form as He. Argon and helium are noble gases or are monoatomic elements.
A molecule can also have even more than 3 covalently bonded atoms. These elements can exist in pure form in other arrangements. A the sharing of electrons between atoms.
Sulphur is a polyatomic molecule as it exists in nature as S6 and S8. Elepsychological sulfur consists of a puckered ring of eight sulfur atoms associated by single bonds. The noble gases are the examples of monatomic gases and they are.
Molecular Chemistry and Molecular Physics. Hence Sulfur is polyatomic. Which of the following elements exists as a diatomic molecule A neon B lithium C.
The sharing of electrons between atoms. These molecules are commonly termed as polyatomic molecules or radicals. A polyatomic ion is also known as a molecular ion that is composed of two or more covalently bonded atoms.
It is a non-metal and its atomic number is 16. Molecules are distinguished from ions by their lack of electrical charge. One particular soap molecule has 18 carbon atoms.
Polyatomic molecules are groups of three or more atoms held together by covalent bonds. Nitrogen exist as diatomic molecule. The polyatomic anion containd hydrogen carbon oxygen.
Hence the correct option is c. Which of the following exists as a polyatomic molecule. Write the name for SnSO42.
B A few facets normally exist as polyatomic molecules which contain more than two atoms. B the transfer of electrons. Ionic compounds is composed of a metal and nonmetal.
Lithium neon carbon phosphorus krypton. But sulphur exists as S8 and hence it is polyatomic. For instance phosphorus exists as P4 tetrahedracontinual polyhedra via four triangular sidesvia a phosphorus atom at each vertex.
It contains 705 carbon 115 hydrogen View more similar questions or ask a new question. There are seven diatomic elements. Click to see full answer.
The elements that have three atoms are called tri-atomic elements. All the others are polyatomic molecules. Diatomic elements are non-metals and exist in pure form as.
The science of molecules is called molecular chemistry or molecular physics depending on the focus. For example oxygen can exist as the triatomic molecule ozone. If the mass of an oxygen atom is 266 x kg and the observed frequency of oscillation is Hz what is the effective spring constant associated with the bond between the.
Selenium Se 8 Ozone O 3 and Phosphorous P 4 some other polyatomic elements.

With Reference To Elements Define The Term Molecule Give Two Examples Each Of A Monoatomic Diatomic Polyatomic Molecule
Solved Problems 2 29 Which Of The Following Diagrams Represent Diatomic Molecules Polyatornic Molecules Molecules That Compounds Molecules That Are Compourds Are Nol Or An Elemental Form Ol Ihe Substance 2 30 Which Of He

Solved 1 Write The Products That Result From The Dissociation Of The Following Ionic Solid In Water Watch For Polyatomic Ions A Kci A Nh Ci A Naoh A D Mgcl A 2 Balance The

Solved 3 Identify The Following Compounds As Either Ionic Or Molecular And Name Them Cacl B Cao Co Ch Nifz Name The Following Compounds That Contain Polyatomic Ion Caco Mgso Al Sojs D

Chapter Exercises Chemical Bonds I Classify Each Of
Molecules Ions And Chemical Formulas
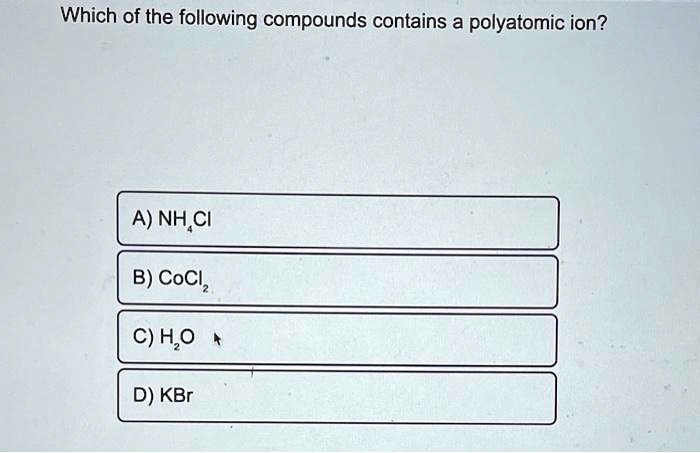
Solved Which Of The Following Compounds Contains A Polyatomic Ion A Nh Ci B Cocl C H O D Kbr

Solved Unit 2 Worksheet 4 Molecular Bonding Review 1 For Chegg Com
Shapes And Polarity Of Polyatomic Molecules Ch 9 46e Studysoup

Solved Question 7 1pts Consider The Lewis Structures For The Compound So3 And The Polyatomic Ions So And So4 Which Of These Must Exist As Sct Of Resonance Structures Soz Only So
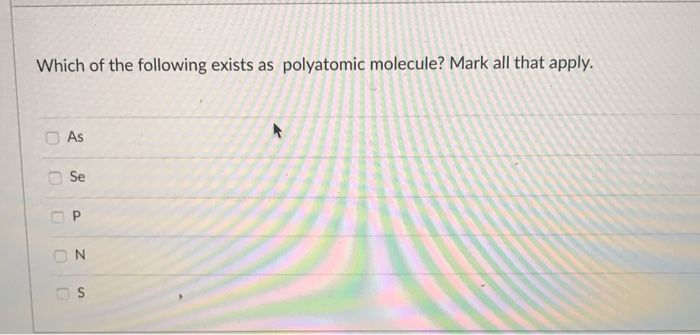
Solved Which Of The Following Exists As Polyatomic Molecule Chegg Com

Solved 1 A Substance That Cannot Be Broken Down Into Chegg Com

Solved 51 In Which Set Do All Elements Tend To Form Anions Chegg Com

Solved Which Of The Following Is A Atomic Element Molecular Element Molecular Compound Ionic Compound Monoatomic Ion Polyatomic Ion Cehuos Co Fel Nhi

Solved Question 64 1 Point Which Of The Following Polyatomic Ions Has Positive Charge Hydroxide Cyanide Phosphate Ammonium Nitrate

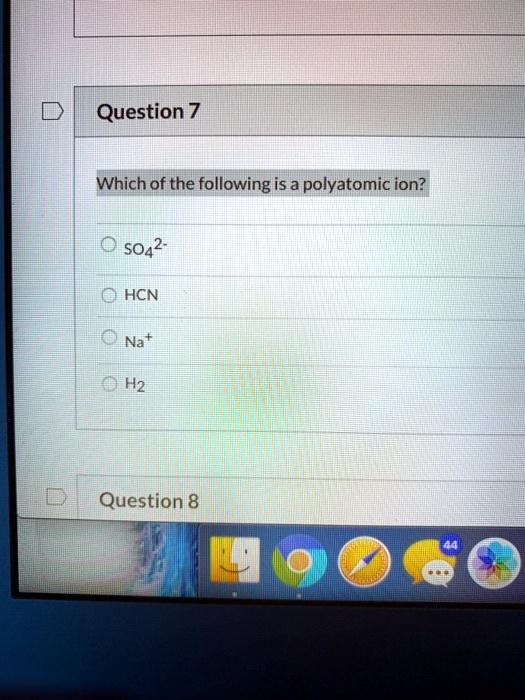
Solved Question 7 Which Of The Following Is A Polyatomic Ion 5042 0 Hcn 0 Na 0 H2 Question 8
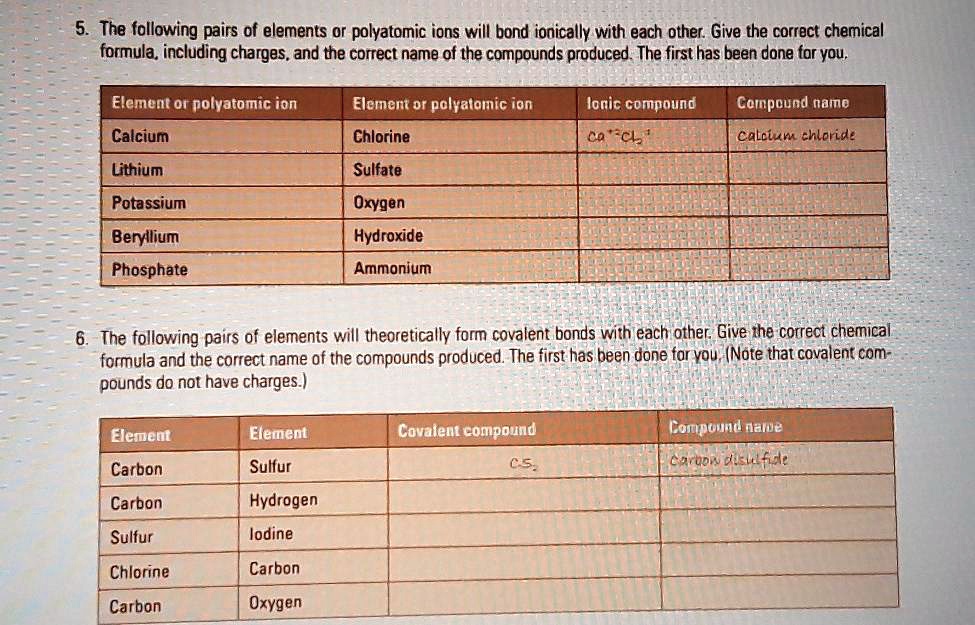
Solved The Following Pairs Of Elements Or Polyatomic Ions Will Bond Ionically With Each Other Give The Correct Chemical Formula Including Charges And The Correct Name Of The Compounds Produced The First Has
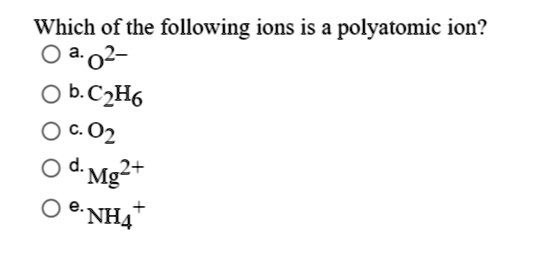
Solved Which Of The Following Ions Is A Polyatomic Ion Oa02 B Cph6 02 Mg E Nh4
Why Are Polyatomic Cations Less Abundant Than Polyatomic Anions Quora
Comments
Post a Comment